Company name: Ansteel Machinery Development Private Enterprise Machinery Factory
Anshan Shuangxing Galvanizing Co., Ltd
Contact: Shen Taisheng
WhatsApp:+8615382137795
Tel.: 0412-8812449
Q Q:1481198998
Postal code: 114000
Email: bestgalvanizing@163.com
Website: www.agxinguo.com
Plant site: Ansteel plant
Office address: No. 111, Ximinsheng Road, Tiexi District, Anshan, Liaoning
As mentioned above, the connection between the wall of the zinc pot, the connection between the bottom and the pot wall is the weak link of the zinc pot, and the connection between the middle of the long side pot wall and the bottom is the weakest link. When a new zinc pot is put into use for the first time, the zinc liquid containing unsaturated lead will cause intergranular corrosion of the pot wall. Under the action of residual stress, thermal stress and hydrostatic pressure of the zinc liquid, brittle cracking may occur. The zinc bath will erode the pot wall, and excessive uneven erosion may lead to the perforation of the zinc pot. Only by understanding and solving these problems can the service life of zinc pot be improved.
1. Corrosion of liquid metal
The corrosion of pure zinc solution on the zinc pot wall is uniform and will not cause intergranular corrosion, as shown in Fig. 1-3a; The iron zinc alloy layer formed by reacting with the zinc wall has a certain protective effect on the pot wall. When the zinc iron alloy layer that plays a protective role just after the zinc pot is put into use has not been formed, do not add lead, but after a period of time after the zinc is completely melted, ensure that a certain thickness of iron zinc alloy layer has been formed on the inner wall of the pot before adding lead.
2. Stress
The main stress causing cracking or deformation of the zinc pot is tensile stress. The tensile stress on the inner wall of the boiler may be caused by the following reasons:
(1) Stress caused by thermal expansion. The thermal expansion coefficient of zinc is far greater than that of steel. If the zinc pot is heated after being packed with zinc ingots tightly, from 20 ℃ to 419.6 ℃ before zinc melting, the expansion of zinc is 12.8mm/m greater than that of steel, and the pot wall will produce tensile stress. This tensile stress can be avoided only by reserving enough expansion space for zinc ingots in the zinc pot.
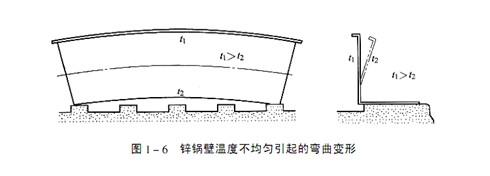
If the zinc pot is heated too fast, the expansion of the upper part of the zinc pot with higher temperature is constrained by the lower temperature bottom, and tensile stress will appear in the lower temperature part. The temperature difference between the inner and outer walls of the zinc pot will cause the inner wall to be in a tensile stress state. The temperature of the zinc pot wall is uneven, causing internal stress, which can cause the zinc pot to bend and deform, as shown in Figure 1-6. The pressure of zinc ingot or liquid zinc on the bottom of the zinc pot will also cause tensile stress at the transition between the bottom of the zinc pot and the side wall.
Correct heating control, slow heating, burner arrangement in proper position, good thermal insulation at the bottom of the zinc pot, temperature difference at various parts of the zinc pot wall controlled below 50 ℃, and these measures can minimize the thermal stress as much as possible. The thermal stress caused by the inconsistent thermal expansion of the heated part and the non heated part of the zinc pot can be controlled by measuring and controlling the temperature difference between them. The maximum allowable temperature difference is related to the size and wall thickness of the zinc pot. The larger the zinc pot, the smaller the maximum allowable temperature difference. For example, the most typical zinc pot is 2.2 meters deep, and the temperature difference is 100 ℃. The macro deformation caused by creep cannot be detected in the zinc pot.
In fact, thermal stress is not the only reason for the damage of the zinc pot, which is often accompanied by liquid metal corrosion.
(2) Hydrostatic pressure of zinc liquid. The hydrostatic pressure of zinc liquid always acts on the zinc pot wall during the use of the zinc pot, which can generate internal stresses such as tension, compression and bending on the pot wall. Therefore, the deep zinc pot wall needs to be supported. The maximum stress area is at the bottom of the side of the zinc pot along the length direction. When the zinc pot operates at high temperature, the material of the zinc pot can creep under the action of stress, resulting in the overall deformation of the zinc pot. The critical stress of creep is related to temperature and time. The outward bending of the pot wall after a long time of use is an example of the creep of the zinc pot material. Because the high temperature tensile strength of steel will decrease with the increase of high temperature service time, the zinc pot will be damaged after a long period of use (about ten years).
(3) The stress generated during the manufacturing process of the zinc pot. In the process of manufacturing the zinc pot, the bending and welding of the steel plate of the zinc pot will produce internal stress. However, modern production technology and methods can reduce these internal stresses very low, which will not have any impact on the use of zinc pot, and no additional measures are needed to eliminate the internal stresses. When the zinc pot is heated to the normal hot galvanizing temperature, the residual stress formed during the processing will be reduced. The zinc pot steel plate must be hot bent during bending to prevent embrittlement due to strain aging.
3. Service life of zinc pot
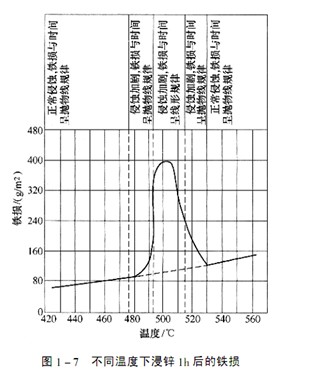
The service life of the zinc pot depends on the speed at which the iron on the pot wall is eroded and dissolved by zinc during use. The speed at the bottom of the zinc pot is the minimum, so the weight lost is the minimum. The amount of iron eroded by zinc can be measured by iron loss. When the zinc pot is put into use, the reaction mechanism of the zinc solution and the pot wall is the same as that of the dip plated parts, that is, zinc reacts with iron to form an alloy layer on the iron surface. With the extension of time, the iron in the boiler wall is continuously dissolved through diffusion. The alloy layer formed on the boiler wall can retard the diffusion. The service life of the zinc pot is related to the iron loss, and the iron loss is related to the interface temperature between the inner wall of the zinc pot and the alloy layer. Figure 1-7 shows the relationship between the iron loss and temperature during zinc immersion/h. When the heating temperature is below 485 ℃, the iron loss increases slowly with the increase of temperature and diffusion, according to the parabolic law. In the temperature range of 490~530 ℃, the iron loss increases linearly with the increase of temperature. The interface temperature between the inner wall of the zinc pot and the alloy layer cannot be measured by general methods. It is the result of a certain balance between the input heat and the output heat of the zinc pot.
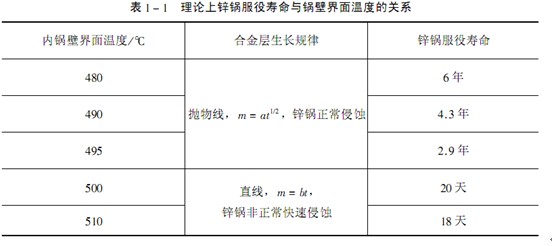
The service life of the zinc pot can be estimated by the time when the thickness of the pot wall is eroded by zinc and the thickness is 20 mm. The estimation is listed in Table 1-1. The iron loss after long time zinc dipping is determined by constant a, which is determined by temperature. When the temperature of the inner pot wall increases, for example, from 480 ℃ to 490 ℃, the service life of the zinc pot will be reduced from 6 years to 4.3 years; When the temperature of the inner wall of the boiler is 500 ℃, the iron loss is linear with the time, and the service life is only 20 days.
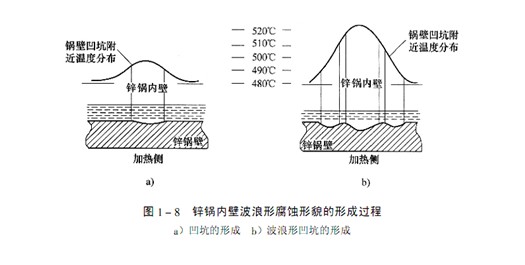
In a certain period of time, the inner wall of the zinc pot is corroded in two kinds of appearance: wavy and relatively flat. If the temperature of the zinc pot wall is uniform everywhere, the thickness of the alloy layer on the pot wall is also uniform, and the appearance of the corroded inner wall of the zinc pot should be a relatively flat plane.
The formation process of wavy appearance can be illustrated in Figure 1-8. For some reason, high iron loss corrosion starts to occur somewhere. For example, at the place where the iron zinc alloy layer is damaged or the place where the inner wall temperature of the boiler reaches the critical temperature due to direct contact with the flame, the thickness of the boiler wall at these places decreases, forming eroded pits, as shown in Figure 1-8a. The reduction of the thickness of the pot wall at the pit leads to the increase of the temperature of the pot wall at the pit, the accelerated erosion increases the iron loss, and the erosion pit gradually becomes larger and deeper. When the temperature in the center of the pit gradually rises and exceeds the critical temperature area, the erosion will slow down, and the strongly eroded high iron loss area will be transferred to the adjacent annular area at the critical temperature, as shown in Figure 1-8b. It needs to be emphasized again that the temperature of the inner wall of the boiler must not be above 480 ℃, nor can it be used for a long time in this temperature range.