Company name: Ansteel Machinery Development Private Enterprise Machinery Factory
Anshan Shuangxing Galvanizing Co., Ltd
Contact: Shen Taisheng
WhatsApp:+8615382137795
Tel.: 0412-8812449
Q Q:1481198998
Postal code: 114000
Email: bestgalvanizing@163.com
Website: www.agxinguo.com
Plant site: Ansteel plant
Office address: No. 111, Ximinsheng Road, Tiexi District, Anshan, Liaoning
The normal or safe operation of the zinc pot means that the stable corrosion rate of the inner wall of the zinc pot is less than 4mm/a during use. If the design and manufacture of hot dip galvanizing furnace and zinc pot are carefully done, and special attention is paid to the temperature measurement and control during heating and operation, the zinc pot generally does not need to be specially inspected during operation, and the replacement period can be determined according to the service time and output.
1. Heating and control of zinc pot
In addition to the structural design of the zinc plating furnace and zinc pot, the heating and control methods of the zinc pot in operation also have an important impact on the service life of the zinc pot. In order to discuss this problem conveniently, the concept of heating intensity is introduced here. The so-called heating intensity refers to how much heat the heat source provides to the boiler wall per unit area in unit time. If the total amount of heat provided is fixed, the longer the heating time is spent or the larger the heating area is, the smaller the heating intensity is; On the contrary, the heating intensity is greater.
The heat input from the heat source to the zinc bath through the zinc pot wall and the heat output from the zinc bath heating the plating parts and the heat dissipation from the zinc liquid surface are in a certain dynamic equilibrium state, and the zinc bath is at a certain temperature accordingly. Only when the dynamic balance is in a proper state, the zinc liquid can maintain the required temperature. When the heat source heats the zinc pot, a temperature gradient will be generated between the inner and outer walls of the zinc pot, which is necessary for heat transfer. There is a functional relationship between the temperature gradient and the heating intensity and the heat transfer coefficient of the steel. The heat transfer coefficient of steel does not change much within a certain temperature range. If it is regarded as a constant, the temperature gradient of the zinc pot wall is a positive linear function of the heating intensity, as shown in Figure 1-5. When the heating intensity increases, the temperature gradient of the boiler wall will increase, and the temperature of the boiler inner wall will increase.
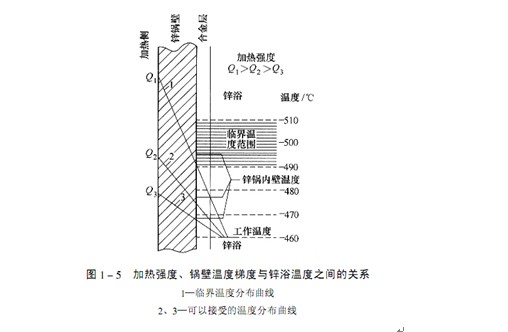
The liquid zinc contacts and reacts with the inner wall of the zinc pot. The effective thickness of the zinc pot wall will be reduced due to reaction erosion. The temperature range of the most violent reaction is 490~530 ℃. Therefore, when heating the zinc pot, the temperature of any local point on the inner wall of the zinc pot should be lower than this temperature range. If the heating intensity is reduced, the temperature of the zinc liquid on the inner wall of the zinc pot and nearby will also be reduced, and the temperature difference with the working area of the zinc pot will be reduced.
The temperature of the inner wall of the zinc pot should be as uniform as possible, and any local point must be lower than 490T, This is what people hope. Although the temperature of the inner wall of the zinc pot cannot be directly measured, if the temperature is often at or above the critical temperature, it can be shown by the increase in the amount of zinc slag generated. Zinc slag is removed from the workpieces in the boiler and the boiler wall ζ Outside the grains, the iron on the inner wall of the pot diffuses through the iron zinc alloy layer into the zinc bath and reacts with zinc to form. The saturation of iron in liquid zinc is 0.035% (at 450 ℃), and excessive iron will make ζ The amount of iron diffused into the zinc bath depends on the temperature of the inner wall of the pot. When the zinc slag formed suddenly increases and no other reason is found, it is basically certain that the interface temperature between the boiler wall and the iron zinc alloy layer is too high. It is suggested to use a chart to draw the output quantity of zinc slag every week (although the amount of zinc slag every week will change, the average error of every 4-6 weeks will not be large). When the amount of zinc slag has an increasing trend and no other reason is found, it can be shown that the temperature of the inner wall of the pot is higher than the critical value, and the extent of zinc corrosion on the pot is greater than normal.
In production practice, in order to improve productivity, it is often hoped to improve the heating intensity of the zinc pot, or even make the zinc pot run under the critical heating intensity. In this way, it is necessary to first understand the allowable critical heating intensity of the zinc pot, that is, when the pot wall does not produce serious erosion, the maximum heat transfer allowed per square meter of zinc pot wall area per unit time. This heat passes through the temperature difference between the critical inner wall temperature of the boiler 490 ℃ and the zinc liquid, so as to achieve the heat transfer coefficient α= 698W/(㎡· K) is transmitted to the zinc liquid. In order to keep the temperature of the zinc bath constant, the heat to be compensated includes: the effective heat required to raise the temperature of the plated part to the zinc temperature, the heat dissipation on the surface of the zinc bath, and the heat required to melt and add zinc ingots. When the zinc bath is constant temperature, the heat input and the heat absorbed by the zinc solution reach the equilibrium condition; According to the temperature of zinc bath and the thermal parameters of liquid zinc and steel, the output of steel plated parts immersed in different zinc bath temperatures is calculated. The following conclusions can be drawn by changing the temperature of zinc bath and the calculation and comparison of the output of plated parts (or the heat supply of zinc pot):
1) The high production rate under high zinc liquid temperature will inevitably lead to the operation of zinc pot above the critical heating intensity, resulting in the rapid damage of zinc pot.
2) Under the limit of normal corrosion rate of zinc pot, the yield at higher galvanizing temperature is lower than that at lower galvanizing temperature.
3) The increase of output makes the ratio of effective heat to heat supply increase significantly.
It can be seen that during the operation of the zinc pot, controlling the maximum output per hour and the appropriate zinc bath temperature is the only way to prevent the zinc pot from being eroded too quickly. During the whole production period, the weight of galvanized pieces per hour and each batch of zinc dipping shall be balanced as far as possible. The total output of galvanized pieces per shift or day shall not be checked and controlled only, so as to avoid overloading of the zinc pot due to the concentration of output in a certain period of time.
In practice, the temperature of zinc liquid should be reduced as much as possible, and the heating intensity of the pot wall should be limited to reduce the corrosion of zinc liquid on the inner wall of the pot. However, the minimum zinc liquid temperature depends on the maximum number of components immersed each time. Too low temperature will make the solvent boil for too long, thus reducing the output, and the coating will be too thick. In general, it is appropriate to keep the temperature of zinc bath at 445~460 ℃.
In the zinc pot being heated, the zinc liquid is constantly moving. It flows upward along the surface of the heated pot wall. When it is close to the surface of the zinc bath, the temperature decreases, so it turns to flow into the middle of the zinc pot, and then flows downward. ζ The grains driven by the zinc liquid will deposit on the pot wall about 100mm below the zinc bath surface. If this is mainly caused by ζ The hard layer gradually becomes too thick and must be carefully scraped off. When adding zinc ingots to the zinc bath, due to the different densities of solid zinc ingots and liquid zinc (7.2g/cm3 and 6.6g/cm3, respectively), the zinc ingots will sink to the bottom of the zinc pot. The dry zinc ingots sink faster than the wet ones, because the wet water vapor on the zinc ingots is heated and vaporized into steam, which will make the zinc ingots move back and forth in the zinc bath. When the zinc ingots touch the pot wall, it will damage the protective iron zinc alloy layer.
2. Remove zinc slag regularly
After the zinc pot is put into operation, zinc slag will be produced continuously. As mentioned above, zinc slag is mainly generated from the reaction of zinc solution and plating parts, zinc solution and steel boiler wall ζ grain. Therefore, the amount of zinc residue is related to the output of hot galvanizing and the technological conditions of hot galvanizing, and is related to the temperature of the inner wall of the pot. ζ The density of grains is only slightly higher than that of zinc solution. ζ The grains are carried by the flowing zinc liquid, and most of them will eventually fall to the bottom of the zinc bath to form zinc slag, except some of them are attached to the zinc pot wall. The thickness of zinc slag shall not exceed 100mm to avoid being stirred by the plating piece, floating in a large amount in the zinc bath and then being absorbed by the plating layer of the plating piece, causing the surface of the plating piece to become rough; Too thick zinc slag layer will also reduce the effective depth of zinc bath in the zinc pot. Long term and excessive accumulation of zinc slag must be prevented. The investigation and study on the zinc leakage accident of some zinc pot perforation shows that the perforation is related to the excessive accumulation of zinc slag on the inner wall of the zinc pot. When a large amount of zinc slag accumulates on the inner wall of the zinc pot, there is no convection of the zinc liquid, and the heat is difficult to dissipate, which causes the temperature of the pot wall to rise, resulting in accelerated corrosion of the inner wall of the zinc pot. If it cannot be found in time, it will quickly develop to perforation. The long-term accumulation of zinc slag will also make the zinc slag firmly adhere to the zinc pot wall, increasing the difficulty of removal. Therefore, when the zinc slag layer is thick, the slag catcher must be used to remove the zinc slag. The interval of slag cleaning depends on the output, usually once a week. The zinc dipping time of the plated parts is short, which can reduce the formation of zinc slag.
3. Disposal of zinc pot when production is stopped for a short time
The heat loss of clean zinc bath surface is about 54000kJ/(m2 · h). When production is stopped for a short time, for example. On weekends, holidays, equipment maintenance, etc., the whole zinc pot shall be covered with a heat insulation cover with good heat insulation performance to reduce heat loss; At the same time, reduce the heating intensity of the zinc pot and maintain the temperature of the zinc bath. Some people think that lowering the zinc temperature after the shutdown can save energy, which is one-sided. During the short-term production stop, the difference between the amount of heat consumed to maintain the temperature of the zinc bath without decreasing and the amount of heat required to reheat the zinc bath to the normal galvanizing temperature after cooling itself is not large when a thermal insulation cover with good thermal insulation performance is added. However, when the temperature of the zinc bath decreases, the solubility of iron in the zinc bath decreases, and the supersaturated iron reacts with zinc to precipitate fine ζ Grain. When the temperature rises, ζ The grains can not be dissolved immediately but suspended in the zinc solution, which will be adsorbed on the coating of the plating piece and make its surface rough.
In addition, if the heating system is cut off to allow the zinc bath to cool naturally, and the corresponding control and alarm systems are stopped, the zinc bath will solidify due to excessive cooling if the control is not properly mastered.
For the zinc plating furnace of refractory brick structure with large fuel heating and heat storage, after the zinc pot is covered with insulation cover, it must be carefully checked to ensure that the temperature of the zinc bath cannot rise too much.
4. Addition of zinc ingot, aluminum and lead
According to the consumption of zinc, an equal amount of zinc ingots must be added to the zinc pot after each shift or two shifts. Do not add a large amount of zinc ingots in a concentrated manner after several days, which will cause a large fluctuation of zinc temperature. A small amount of zinc ingots can be added manually, and a large amount of zinc ingots must be added with a crane or other suitable machinery. If more zinc ingots need to be added at the same time, they should be evenly distributed along the entire length of the zinc pot.
Aluminum in zinc bath can reduce the formation of zinc ash and make the coating bright. The maximum mass fraction of aluminum in liquid zinc shall not exceed 0.02%. Too little aluminum will make the surface of the plated parts have yellow color, while too much aluminum will cause leakage and accelerate the corrosion of the zinc pot. Zinc aluminum alloy shall be regularly added to the zinc pot.
The maximum amount of lead dissolved in zinc solution is 1.2% (mass fraction) at 450 ℃. The density of lead is higher than that of zinc. Adding excess lead will sink to the zinc bath. In the pure zinc bath, adding lead and maintaining its mass fraction above 0.6% is conducive to the zinc slag sinking into the bottom of the pot. When the lead ingot is put into the zinc bath, it will directly sink to the bottom of the zinc bath and gradually melt. Most of the lead ingots do not dissolve into the zinc bath, but penetrate into the zinc slag layer and accumulate below the zinc slag layer. When the zinc slag is removed, part of the lead will be taken away with the zinc slag. Therefore, it is better to add lead into the zinc bath by dispersing and adding fine lead particles regularly.